HCL is written wrong and probably a typographical error. Answer 1 of 2.
 |
| Ikatan Kovalen Mengenal Jenis Jenis Dan Contohnya |
In general if the electronegativity difference between two atoms is less than 05 the bond is considered nonpolar even though the only truly.
. This value lies between 04 to 20 which indicates that the bond. So for HCl the electronegativity difference ΔEN 316 22 096. Two charge formations will go on there. What is considered non polar.
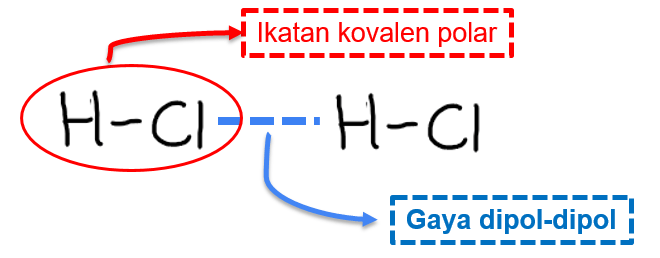
HCl is the polar molecule in chemistry. This is because the Chlorine Cl atom in the HCl molecule is more electronegative and does not share the bonding electrons equally with Hydrogen H. HCL is neither polar or non-polar. HCl is a polar molecule.
Chlorine is near getting a partial negative. HCl also known as Hydrogen Chloride is a gas at STP. In HCl chlorine is highly collated to a hydrogen atom. Hydrochloric Acid is extremely polar as it has an asymmetric center meaning that it doesnt have an even number of electrons making it very acidic with a pH level close to zero.
The difference between electronegativity values of hydrogen and carbon is small and thus C-H bond is non-polar. Electronegativity of Chlorine Cl 316. Therefore we do not draw any dipole arrow for C-H bonds.
 |
| Polar Covalent Bond An Overview Sciencedirect Topics |
 |
| Hcl Intermolecular Forces Type Strong Or Weak Techiescientist |
 |
| Polar And Non Polar Covalent Bond Chemical Bonding And Molecular Structure Chemistry Class 11 |
 |
| Give Reasons Hcl Is Polar But H2 And Cl2 Are Non Polar |
 |
| Give Reasons Hcl Is Polar But H2 And Cl2 Are Non Polar |
Lokasi:







Posting Komentar untuk "hcl polar or nonpolar"